Inhaled Insulin Recommended For FDA Approval
New method seen as practical alternative for several diabetics
October 5, 2005
WASHINGTON, DC—On Thursday, a federal health advisory panel recommended FDA approval of a new inhalable form of insulin, offering several of the nation's 18 million diabetics a chance for better control of their sugar levels.
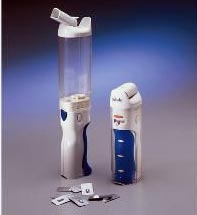 |
Exubera®, with Exubera® Bong |
|
|
Exubera®, which will be produced by Pfizer, Sanofi-Aventis, and Nektar Therapeutics, is a rapid-acting, dry-powder type of insulin that will be delivered to the lungs via a special bong-shaped inhaler device, one whose simplicity and ease of use has been lauded as “not any more complicated than what diabetics rely on now.”
“This is great,” said Ed Martinez, a 43-year-old Type II diabetic who lives in Bayside, NY. “I’ve been waiting years for something like this. I mean, I’ve been working hard on my diet and exercise and all, but my diabetes has gotten to the point where I need to take the ‘fast’ insulin before I eat, but not that bad where I gotta take the ‘long-acting’ insulin too. And that’s a good thing, because I’m totally scared of needles, but not so scared where I can't do the fingerpricks
for the sugar testing.”
Like approximately ten or eleven other diabetics nationwide who appear to be appropriate candidates for this new inhaled form of insulin, Martinez also does not smoke, have asthma, chronic bronchitis, or any other known pulmonary condition. Also, he is immune to pathogens that could cause mucus to accumulate in the lungs, such as the common cold virus, other viruses, and all currently identified strains of S. pneumoniae, H. influenzae, and M. pneumoniae.
 |
Ed Martinez |
|
|
Most importantly, potential Exubera® users like Martinez must exhibit a complete lack of concern or awareness about the potential long-term effects of inhaling a powdered form of any substance into the lungs, let alone a powerful anabolic hormone like insulin.
Other chronic inhalations, such as those of dust, asbestos, talc, and silica, have been shown to lead to debilitating and irreversible conditions known as “pneumoconioses.” But while Pfizer, Sanofi-Aventis, and Nektar plan to conduct studies of long-term effects until 2019, the rush to get Exubera® onto the market as soon as possible is “entirely justified,” say company representatives.
“We can’t be wating fifteen, twenty years to put things like this on the market,” said Stacey Carroll of Pfizer. “It’s a $3 billion insulin and injector market, right now.”
As for Mr. Martinez, he remains eager to achieve better glucose control. “Pneuma-coney-WHAT?” he says, with a laugh. “Yeah, whatever! I’m just ready to start inhaling my insulin, so the sooner it’s available, the sooner I’m gonna get my doc to prescribe it.”
FDA approval is anticipated by January 2006, and Exubera® is expected to be officially released by midyear.
|